Effects of EM waves in tissue
- Muzamil Arshad

- Sep 1, 2020
- 6 min read
Quick recap from previous post:
1. Classical (that is as described by Maxwell's equations) electromagnetic (EM) waves are described as continuous oscillation of the electric and magnetic fields which travel as the speed of light (in vacuum) ~ 3 x10^8 meters/sec or ~186,282 miles/sec
2. The energy of the EM wave is given by the equation: E =hf
h = Planck's constant ; f = frequency of EM wave
What are the effects of EM waves in tissue?
We are all exposed to EM waves on a daily basis. There is basically no way around this (you could live in a Faraday cage I suppose!). There are radio-waves around us from AM/FM radio, cell towers, satellites. There is the 'ultra-violet' waves we are exposed when we are outside on a sunny day (and should wear sunscreen on exposed skin). There are EM waves from medical imaging (X-ray, CT, MRI, PET scans) and of course the fact that you can SEE is a result of EM waves being detected by your retina!
I think the most convenient way of understanding the effects of EM waves on humans is to classify them as : "Ionizing Radiation" or "Non-ionizing Radiation"
What is Radiation?
First, a brief statement on the word 'radiation'. This word, in my experience, always worries people. They immediately think of a nuclear disaster or a fish with three eyes!

The word 'radiation' simply means the transmission/emission of energy through space in the form of either particles or waves. So while no one worries about getting an ultrasound (for example an OB ultrasound to check fetal development), ultrasound is IN FACT radiation! An ultrasound transmits energy in the form of a mechanical waves. Namely the tissue through which ultrasound travels compresses and decompresses as the US travels through it (Figure 1). Actually ultrasound waves are NO different than the mechanical waves responsible for hearing! Ultrasound waves are just higher frequency waves and we cannot hear them! If you were in the vacuum of space (where there are basically no particles to compress and relax) no one would hear you speak (Figure 2)!!
Bottom Line: Radiation does NOT mean RADIOACTIVE.
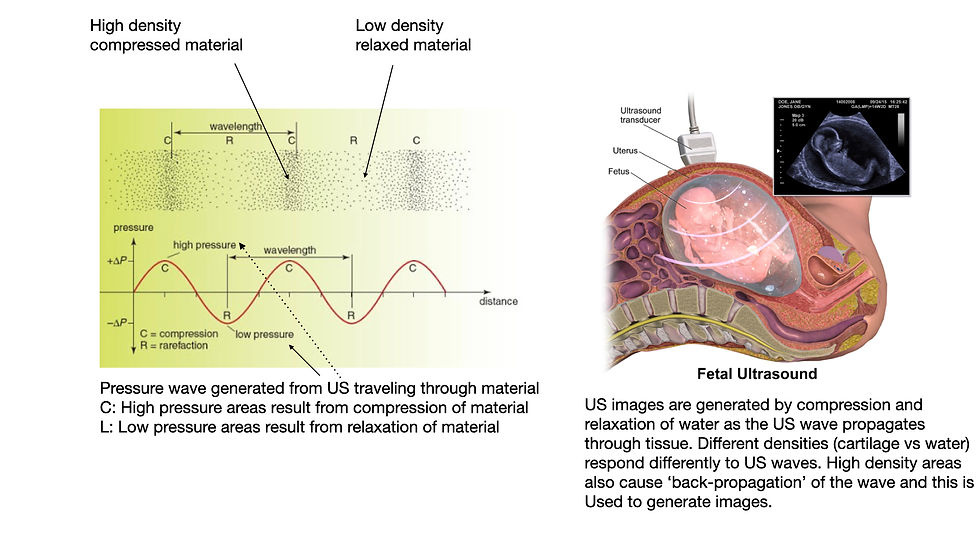
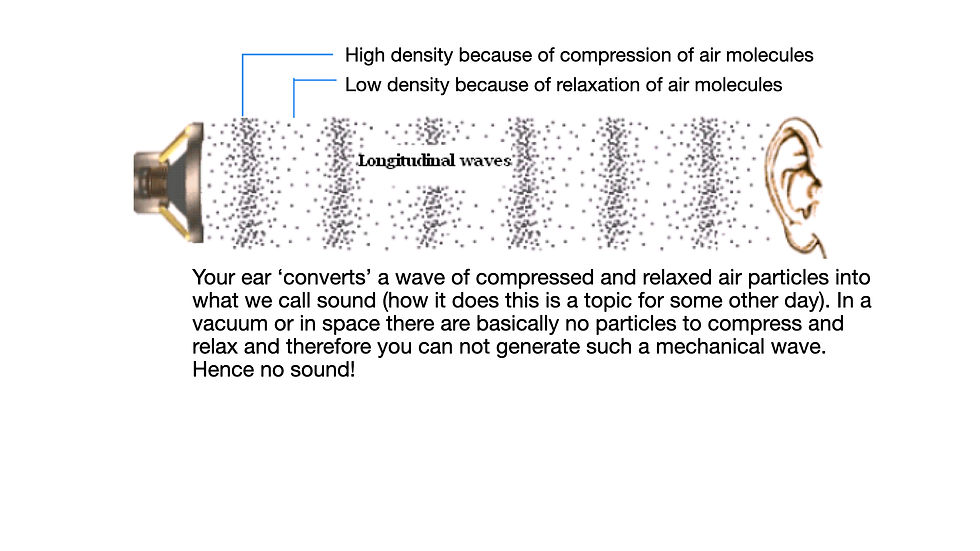
What is ionizing radiation?

When we talk about ionizing vs non-ionizing radiation, we are referring specifically to radiation of the Electromagnetic energy (EM Waves). Non EM radiation (Figure 3) is not ionizing, therefore, we do not worry about its effects on tissue (MRI and US are generally considered safe in children and pregnant patients. Caveat here is that at high magnetic field strength, there are theoretical concerns about radio-waves used in MRI and heating tissue and therefore potentially causing thermal injury. Still this is not ionizing). Figure 3 demonstrates that electromagnetic waves can be classified as ionizing or non-ionizing.
Note that high frequency, and therefore high energy, EM waves are ionizing. So what is ionizing radiation? First, recall what an ion is. An ion is an atom or molecule that is charged (that is a positive or negative charge). Figure 4 depicts the most common ionization that occurs in biological tissue exposed to ionizing radiation. Before, describing an ionization event, you maybe wondering why is that high energy EM radiation is needed. Note that ionization requires removing an electron from an atom or molecule. This process requires energy (an analogy would be if you wanted to remove an object from the earth you would need to provide energy to overcome the gravitational attraction between the object and the earth). How much energy is needed to ionize water? About 14.53 eV (electron volts). Using the equation E=hf (and appropriate unit conversions), the frequency of the EM wave that corresponds with this ionization energy is 3.47 x 10^15 Hz. The ultraviolet range of the EM spectrum corresponds to ~10^14 - 10^16 Hz. So, look at what we found! The energy needed to ionize water is contained within the UV range. If you look back at Figure 3 you will note that radiation is considered ionizing right at the UV range. As an interesting side note you maybe wondering why is it that when we talk about sun screen, the experts always tell you that you need to block UVB. This is because, not all of the UV spectrum is ionizing. Frequencies below 10^15 Hz won't ionize (but contribute to skin aging etc so should still want to block!).
Now that we have defined an ion and how much energy is needed to ionize we will discuss what happens during an ionization event and why we care. We will focus on the effects of ionization in the human body, which is primarily mediated through water (we are after all mostly water). A detailed understanding of ionization energy does require some knowledge of general chemistry (think back to undergraduate Gen chem!) and I will not go into that on this post (perhaps some other time). In simple terms recall that water, H2O, is composed of oxygen, which forms covalent, chemical (meaning mediating by electrons), bonds with two hydrogen atoms. As shown in Figure 4, the covalent bond between Hydrogen and Oxygen consists of two shared electrons. When an EM wave of sufficient energy (>= 14.53 eV) is absorbed by an electron in water molecule, that electron is ejected from the molecule. The electron carries a negative charge, and therefore the remaining molecule of water is positively charged (hence ionization!). This electron and H2O+ molecule re-arrange to form a hydroxide ion and proton.
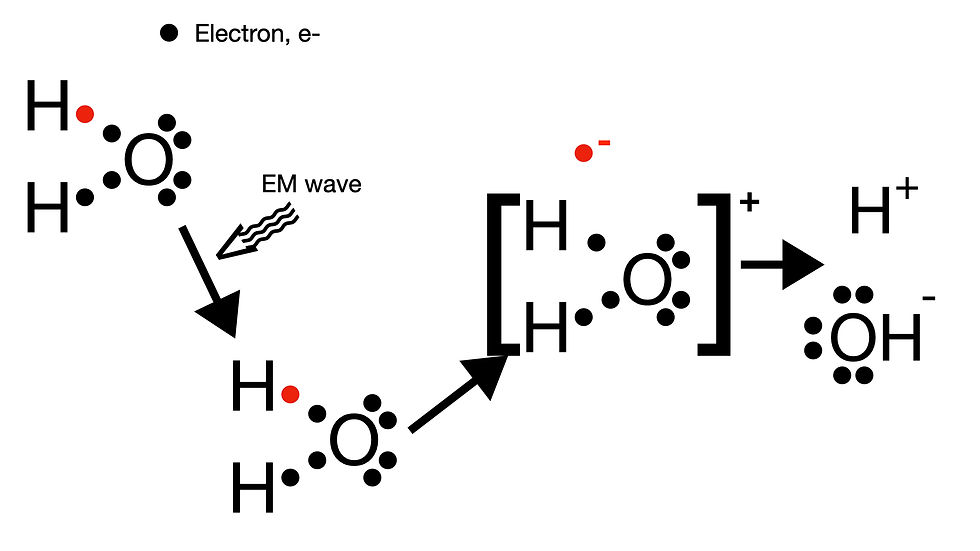
Why do we care about ionizing radiation?
We have spent quite some time on describing radiation, ionizing vs non-ionizing EM radiation and the most common ionizing interaction with water in the human body. Finally, why do we care about ionizing radiation? Arguably the most important part of a cell is its DNA. Damage it, and the cell will die or the resulting mutations in the DNA will cause cancer. Because DNA is a molecule (with various covalent bonds with other atoms mediated by electrons) having charged "free" (unbound) electrons and charged hydroxyl radicals near DNA is unsafe as these chemicals are highly reactive! In fact cells have various molecules whose job it is to 'capture' these free electrons and other reactive molecules (by the way it should be noted that DNA "damage" occurs spontaneously simply due to 'errors' in biology, but mutagens such as ionizing radiation and other chemicals increase the frequency of DNA mutations or damage.)
To sum up, ionizing radiation for biological tissue is dangerous because the ionized molecules are highly reactive and can damage the integrity of DNA. Not all cells are equally sensitive to radiation. Cells that are rapidly dividing are more sensitive than cells which are not (GI tract and bone marrow are very sensitive to even 'low doses' of radiation). Bacteria and virus on the other hand tend to be more resistant than human cells.
I must emphasize that we should not be unnecessarily 'afraid' of ionizing radiation. If you have trauma with possible fracture you should get an X-ray! Furthermore, ionizing radiation is a powerful weapon in the fight against cancer! As stated, while radiation can increase the risk of developing cancer decades down the road (but so can chemotherapy and other chemical exposures) it can also be used to kill cancer cells. In fact, about 50-66% of cancer patients will get radiation at some time (more on this topic in the future). As a disclosure, I am a resident in Radiation Oncology.
What are the effects of non-ionizing EM radiation on tissue?
Very briefly, the effects of ionizing EM radiation on tissue is related to thermal energy. Namely this type of EM radiation can induce currents and also cause increased motion of water molecules, translating to higher temperatures. While non-ionizing radiation does not damage DNA, this does NOT mean it cannot cause harm. In fact, a well established method for treating tremors uses highly focused ultrasound (which is non-ionizing) to heat up and destroy brain tissue (Figure 5). It should be noted that while non-ionizing radiation does not damage DNA it certainly can destroy tissue.

In fact, even microwaves (non-ionizing EM radiation) which do not damage DNA, could potentially cause harm. Microwaves cause water molecules to vibrate vigorously, hence heating up water. Recall, we humans are mostly water. So if you were exposed to microwaves you could boil (the discovery that microwaves could be used to heat up food was an accidental discovery)! As a matter of fact, Microwaves have a metallic mesh on the door to prevent the 'leak' off microwaves (Figure 6). For you Batman fans (Batman Begins) recall Ra's al Ghul's weapon of choice to destroy Gotham was ...................... A Microwave Emitter!!

Summary
Radiation simply means the transport of energy through space via waves or particles (eg. mechanical wave like an ultrasound or electromagnetic waves).
Electromagnetic (EM) waves can be ionizing or non-ionizing.
Ionizing EM waves primarily effect biological tissue by ionizing water, and EM waves of high frequency (therefore energy) in the UV range and above are needed.
Ionizing radiation can damage DNA, though not all cells are equally sensitive.
While ionizing radiation can increase risk of cancer, it is used to effectively treat cancer as well!!
Non-ionizing radiation (like microwaves), which does not damage DNA, nonetheless can cause severe tissue damage (focused ultrasound for ablation)
Thanks for reading.



Comments